Abstract
Introduction: Systemic light chain (AL) amyloidosis is a clonal plasma cell disorder characterized by deposition of misfolded immunoglobulin light chain products in vital organs, causing their dysfunction. Identifying the molecular mechanisms underlying AL amyloidosis pathogenesis as well as novel therapeutic targets for these patients is needed to enhance treatment availability and proper treatment selection.
MicroRNAs (miRNAs) are short, non-coding RNAs that regulate gene expression and have a role in cancer development and progression. miRNA-mRNA interactions can determine the molecular mechanism involved in AL amyloidosis pathogenesis and may suggest novel therapeutic approaches. To date, knowledge about circulating or bone marrow (BM) miRNAs involved in AL amyloidosis is lacking.
Objectives: To decipher miRNA-mRNA interactions that are involved in AL amyloidosis pathogenesis and to identify biological pathways, regulated by miRNAs, as novel therapeutic target in AL Amyloidosis.
Methods: miRNA and mRNA expression profiles were determined using the NanoString nCounter assay and RNA-Seq, respectively. Detection of potential miRNA targets, and enriched biological pathways was performed by the bioinformatics tools Ingunity Pathway Analysis (IPA) and DIANA miRPath. MiRNA and gene expression profiles were validated by qRT-PCR in 50 AL, 50 MM and 10 healthy controls (HC) samples. The enriched biological pathways identified were inhibited by using BCL2, PI3K and MEK inhibitors. The effect of aberrantly expressed miRNAs on potential molecular targets was analyzed in ALMC-1 cells by transfecting the cells with miRNA mimic, following proliferation assay (WST1), qRT-PCR, Western blot analysis mitochondrial depolarization assay, cell cycle analysis and Annexin-PI staining.
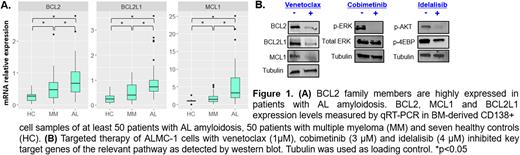
Results: BM and plasma miRNAs were differentially expressed in AL compared with MM patients or HCs. The differentially expressed miRNAs and mRNA in AL patients regulate key signaling pathways including mitochondrial dysfunction, NFkB signaling, activation of MAPK and PI3K/AKT pathways, all linked to cancer cell growth and proliferation, therefore may be used as a therapeutic target. Particularly, genes related to the mitochondrial activity (BCL2, MCL1, and BCL2L1) were upregulated in AL patients (Figure 1A). Upregulation of miR-9-5p and miR-181a-5p (by transfecting an AL amyloidosis cell model with miRNA mimics), led to decreased BCL2, MCL1 and BCL2L1 oncogene expression, followed by depolarization of mitochondrial membranes and increased apoptosis. Moreover, treatment with venetoclax, a BCL2 inhibitor, resulted in upregulation of these miRNAs followed by downregulation of BCL2 family members (Figure 1A), suggesting that venetoclax directly inhibits BCL2 and indirectly affect miRNAs that regulate anti-apoptotic proteins. This finding supports the use of BCL2 inhibitors in AL amyloidosis. Finally, we identified a subset of miRNAs in AL patients that regulate the PI3K and MAPK signaling pathways. Treatment with PI3K inhibitor (Idelalisib) and MEK inhibitor (Cobimetinib) resulted in downregulation of pAKT and pERK in ALMC-1 cells (Figure 1B) and inhibition of cell proliferation. Moreover, we found that AKT and ERK target genes were highly expressed in BM samples of pre-treated AL patients as compared to HCs. These target genes were downregulated following treatment with PI3K and MAPK inhibitors in ALMC-1 cells, suggesting a functional biological relevant to inhibit these pathways in AL patients.
Conclusions: We provide new data on cellular regulation in AL amyloidosis mediated by miRNAs and the aberrant expression of oncogenic/tumor suppressor genes, which are not related to DNA point mutations. As BCL2 inhibitors are becoming an important therapy for AL amyloidosis, this work lays the molecular foundations for this type of treatment. miRNA-mediated signaling pathways involved in AL amyloidosis, such as PI3K and MAPK, may assist in tailoring more specific treatments.
Disclosures
Muchtar:Protego: Consultancy; Janssen: Honoraria. Dispenzieri:Janssen: Membership on an entity's Board of Directors or advisory committees; Oncopeptides, and Sorrento: Other: Data monitoring safety committee; Alynlam, Pfizer, Takeda, and BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal